Study on Toughening Polylactic Acid with Dynamically Crosslinked Epoxidized Soybean Oil Cured Network
- Share
- publisher
- hoey
- Issue Time
- Aug 16,2024
Summary
This study enhances PLA toughness by crosslinking ESO and citric acid. The PLA/VEC blend remains biodegradable in 30 days while retaining properties after recycling.

Study on Toughening Polylactic Acid with Dynamic Crosslinked Networks
Traditional petroleum-based polymers face threats from environmental issues and resource depletion, while polylactic acid (PLA), due to its biodegradability and excellent properties, has been widely used in various fields. However, the brittleness of PLA limits its application. Current toughening methods, such as plasticizer modification and chemical copolymerization, have limitations. Therefore, recent research has focused on enhancing PLA toughness through dynamic crosslinked networks.
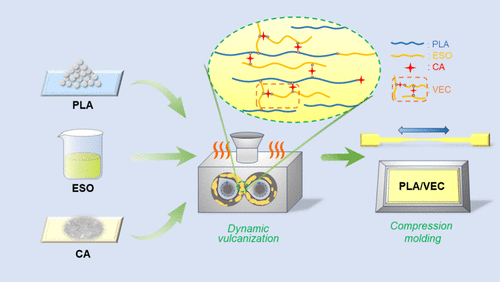
Dynamic Sulfurization of PLA/VEC Blends
PLA and epoxy soybean oil (ESO) were mixed at 170°C and 60 rpm until uniform, followed by the addition of a mixture of anhydrous citric acid and 5 mol% zinc acetate. The mixture underwent dynamic sulfurization for 10 minutes and was then molded using a compression molding machine. The fixed amount of VEC (dynamic crosslinked network formed by ESO and citric acid) was 5 phr. By varying the carboxylic acid/epoxy resin equivalent ratio (R values: 0.1, 0.3, 0.5, 0.8, 1.1), the effect on PLA toughening was studied, resulting in blends labeled PLA/VEC0.1-5 to PLA/VEC1.1-5.
Reaction Mechanism
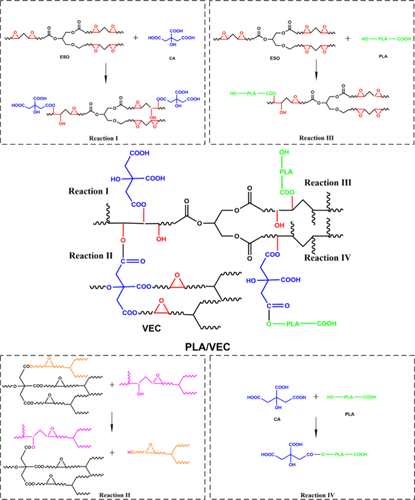
During dynamic sulfurization, PLA reacts with ESO and citric acid (CA) through complex reactions. The highly reactive epoxy groups and carboxyl groups form ester bonds and new hydroxyl groups through ring-opening reactions (Reaction I). Ester exchange reactions within VEC (Reaction II) occur under thermal activation, promoting topological flow. PLA terminal carboxyl groups react with epoxy groups of ESO (Reaction III), enhancing chemical compatibility, while PLA terminal hydroxyl groups react with CA carboxyl groups through esterification reactions (Reaction IV), extending, crosslinking, and compatibilizing PLA molecular chains, thus improving material performance.
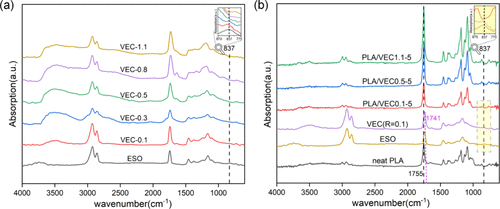
Structural Analysis of PLA/VEC Blends
Infrared spectroscopy analysis shows that as the carboxylic acid/epoxy equivalent ratio (R value) increases, the hydroxyl stretching vibration peak (3483 cm⁻¹) significantly increases, while the epoxy group absorption peak (837 cm⁻¹) gradually weakens. In the PLA/VEC blends, the hydroxyl peak at 3483 cm⁻¹ from VEC is absent, indicating that hydroxyl groups generated from the ring-opening reactions of ESO and CA, as well as inherent hydroxyl groups of CA, participated in the dynamic sulfurization and ester exchange reactions with PLA macromolecular chains.
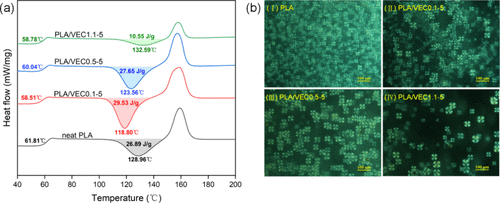
Thermal Properties Analysis
DSC curves show that due to the ring-opening reactions between carboxyl and epoxy groups and ester exchange reactions between hydroxyl and ester groups, the rigidity of PLA molecular chains is moderately reduced, resulting in a slightly lower Tg for PLA/VEC compared to pure PLA. VEC acts as a "nucleating agent" in the PLA matrix, making the cold crystallization peak of PLA/VEC blends narrower and sharper, leading to a decrease in Tc. POM analysis further reveals that PLA/VEC blends exhibit a more saturated crystalline morphology, indicating that the addition of VEC enhances the crystallinity of PLA.
Rheological Analysis
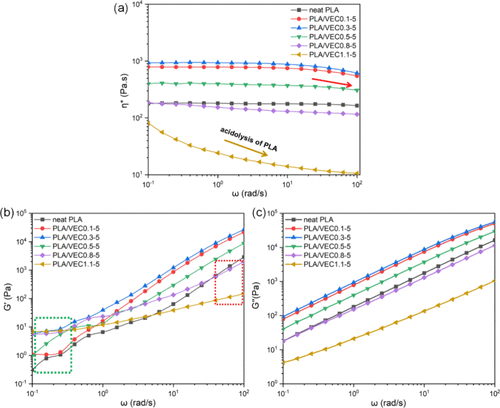
The rheological behavior of PLA and PLA/VEC blends shows that with increasing R values, the complex viscosity of the blends initially increases and then decreases. At lower frequencies, the storage modulus of PLA/VEC blends exceeds that of PLA, attributed to the crosslinked structure enhancing entanglement, resulting in greater melt elasticity. In the mid-frequency range, the blends exhibit Newtonian fluid-like behavior. However, at higher frequencies, blends with R values of 0.1 and 0.3 exhibit more pronounced shear thinning compared to PLA. This is due to the crosslinked structure exacerbating the degree of entanglement. As R value increases, the loss modulus initially increases and then decreases, reflecting a reduction in molecular chain mobility. This confirms that the network crosslinked structure introduced by VEC reduces molecular chain mobility.
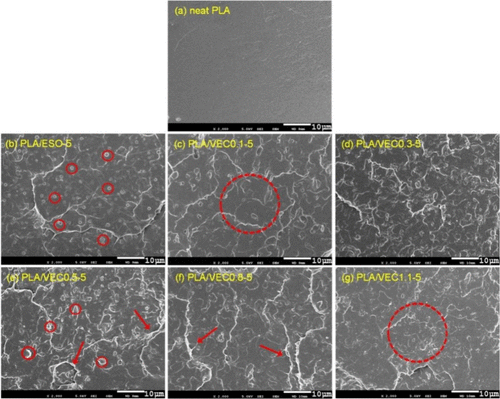
Morphological Analysis
SEM analysis shows that pure PLA has a smooth and flat fracture surface, indicating brittle fracture characteristics. The fracture surface of pure PLA/ESO blends exhibits distinct ESO particles, suggesting poor compatibility of ESO in the PLA matrix. In contrast, the fracture surface of PLA/VEC blends shows varying degrees of roughness, indicating good interfacial adhesion between PLA matrix and VEC. The ring-opening reactions between ESO and CA generate new chemical bonds, including ester exchange and esterification reactions between VEC and PLA matrix. This significantly enhances VEC's compatibility and interfacial bonding within PLA.
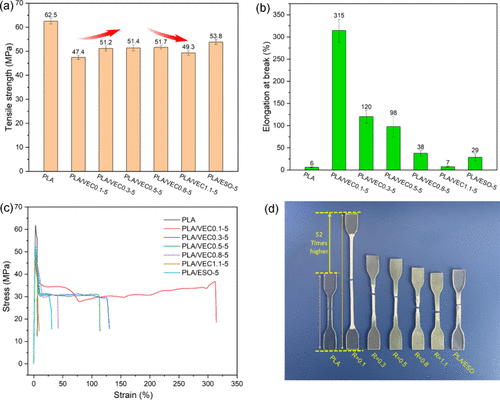
Mechanical Properties Analysis
Tensile performance analysis of PLA and PLA/VEC blends shows that, at 5 phr addition, both PLA/VEC and PLA/ESO blends have lower tensile strength than pure PLA. However, the elongation at break of PLA/VEC blends significantly improves, reaching 315% at an R value of 0.1, showing remarkable toughness. The reduction in tensile strength of PLA/VEC blends can be attributed to the plasticizing effect of ESO. Additionally, the presence of VEC increases the spatial separation between PLA molecular chains, reducing the interaction forces between long PLA chains. This effect outweighs the direct plasticizing and toughening effect of ESO on PLA.
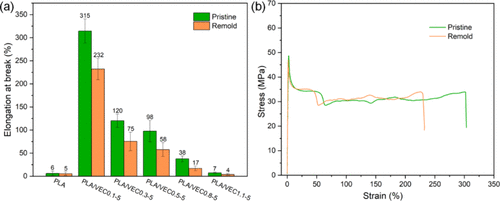
Recyclability
The PLA/VEC blends were shredded and remolded into films. The elongation at break of the recycled blends decreased, with PLA/VEC0.1-5 elongation reducing from 315% to 232%. Despite the reduction in toughness after recycling, it still remains excellent. Testing the degradation behavior of PLA and PLA/VEC in alkaline solutions showed that all samples completely degraded within 30 days, with residual weight initially increasing and then decreasing with R value. Infrared spectroscopy analysis indicates that degradation primarily occurs through ester bond cleavage.
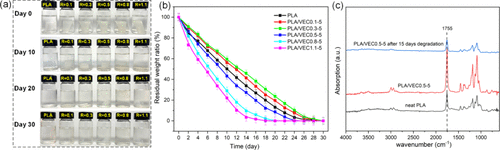
Conclusion
In this study, epoxy soybean oil and citric acid were used to create a zinc acetate-catalyzed epoxy/carboxylic acid exchange network with PLA as the matrix. Due to its inherent crosslinked network structure, VEC exhibits good compatibility and promotes chain reactions within PLA macromolecular chains. Among the PLA/VEC blends, PLA/VEC0.1-5 demonstrates the highest toughness and impact strength, with an elongation at break of 315% and an impact strength of 28.5 kJ/m², which are 52 times and 1.8 times that of pure PLA, respectively. Although the toughness of recycled PLA/VEC blends decreases to some extent, they still show advantages over PLA. Additionally, PLA/VEC blends retain PLA’s green biodegradability, completely degrading within 30 days in alkaline environments.